Tropospheric Gasphase Chemistry (Summer Smog): An overview
Johannes Staehelin
Institute for Atmospheric and Climate Science (IACETH), Swiss Federal Institute of Technolgy Zurich (ETHZ)
Universitatstrasse 16
CH-8092 Zurich, Switzerland
email: Johannes.Staehelin@env.ethz.ch
(including pictures provided by A.S.H. Prevot (PSI))
1. Introduction: Atmospheric Ozone
Ozone sonde measurements of Payerne (Switzerland)
|
Figure 1. Ozone concentration [mPa] |
![Ozone concentration [mPa]](Ris/sum_sch/ris_sta_1.jpg) |
- black: 1970
- red: 1980
- green: 1990
- blue: 2000
Notes to Figure 1
Ozone measurements have been performed regularly from light balloons since the late 1960 launched from Payerne in the Swiss plateau (Fig. 1). The measurements illustrate that (a) ozone concentrations are much larger in the stratosphere than in the troposphere; (b) ozone concentrations have decreased in the stratosphere (because of ozone depletion by anthropogenic emissions of ozone depleting substances such as chlorofluorocarbons); (c) ozone in the troposphere has increased during the last decades.
Ozone is an important air pollutant in the troposphere produced in summer smog and also a very efficient greenhouse gas (especially in tropopause region).
|
Figure 2. Solar spectrum outside the atmosphere and at Earth‘s surface |
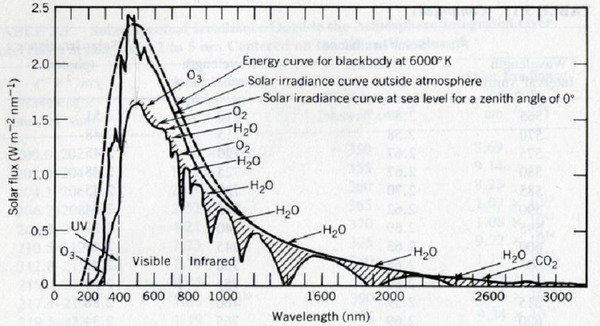 |
Notes to Figure 2 (from Seinfeld and Panis,1998, p.28): UV-radiation in the troposphere
The radiation of the sun is similar to the radiation emitted by a black body at 6000 K (black body radiation, dotted line). Because of solar physical processes and other processes in the interstellar room the curve shown in black reaches the Earth‘s atmosphere. The solar spectrum is significantly changed when passing through the stratosphere: The short wave radiation is entirely absorbed by stratospheric ozone and molecular oxygen below approximately 300 nm and therefore only photochemical reactions requiring radiation above 300 nm take place in the troposphere. The visible radiation is much less absorbed passing through the atmosphere. Thus, photochemistry in the troposhere is driven by solar UV(and visible)-radiation with wavelengths between approximately 300 and 600 nm.
In the infrared part of the spectrum water vapour and carbon dioxide (and some other greenhouse gases are significant absorbers.
|
Figure 3. System of stratospheric and tropospheric gas phase chemistry: Radical chain reaction |
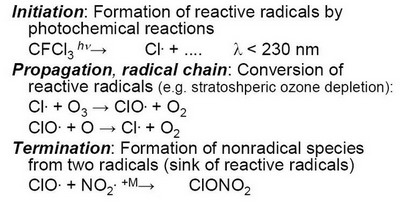 |
Notes to Figure 3: Principles of atmospheric gas phase chemistry
Stratospheric as tropopsheric gas phase chemistry include a variety of individual reactions. They can be viewed as radical chain reactions, which include the following type of reactions (Fig. 4.3 illustrates the principles of stratospheric chemistry):
Initiation reactions. They are photochemical (photolysis) reactions, driven by solar light producing reactive radicals.
Propagation or radical chain. The radicals produced by the initiation reactions are very reactive, reacting with most (reactive) molecules. By sequences of radical reactions the same radicals are formed again, leading to a radical chain. Such radical chains are very efficient because the radicals are reformed, e.g. one chlorine radical formed from one CFCl3 depletes not only one O3 but many O3 molecules since Cl is reformed by the reaction of ClO with O.
Termination. If one radical reacts with another radical, less reactive non radical species are usually formed. These reactions stop the radical chain and therefore limit the yield of the radical chain.
In the atmosphere the systems are more complex, because the molecules formed in the termination reactions can be activated again or different radical chains interact with each other.
Overview of presentation
2. Ozone (photooxidant) formation
2.1. Photostationary state
2.2. ROx-radical chain
2.3. Most important termination reactions
2.4. Ozone destruction in (very) clean air
3. Oxidation during night
4. Limitation regimes
5. Maximal O3 concentrations reported from PBL in urban plumes
2. Ozone (photooxidant) formation
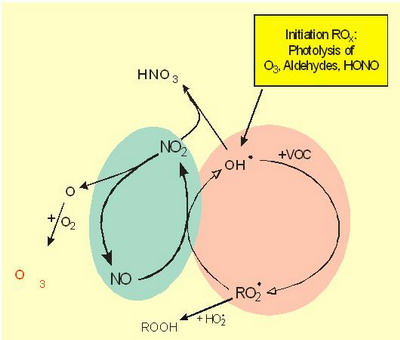
|
Two coupled radical chain reactions: NOx (green): NO, NO2, ROx (red): OH., HO2., RO., RO2. |
|
Figure 4. Principles of tropospheric gas phase chemistry |
Notes to Figure 4: Tropospheric gas phase chemistry: Overview
Tropospheric gas phase chemistry includes two (connected) types of radical chains.
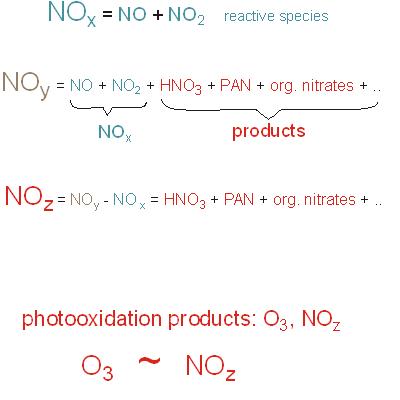
The nitrogen oxides (NOx: NO + NO2, green; they are radicals, but not characterized by radical point in the following). Nitrogen oxides enter the system mainly by emissions from fuel combustion (no initiation reaction).
NO2 is the precursor of tropospheric ozone.
The ROx/HOx radical chain reaction system (red). They are produced by photolysis. The ROx-radical and the NOx radical chains are connected (see below).
The yield of the reaction system is limited by the following termination reactions. The (most important) termination reactions are: One type includes RO2 and/or HO2 radicals (forming peroxides) and another includes OH and NO2 (forming HNO3).
2.1.Photostationary state
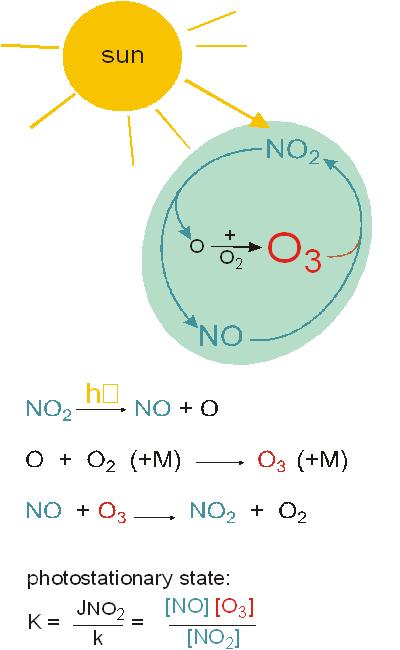
|
Photostationary state: fast equilibrium:
left: Figure 5
Definitions:
|
Notes to Figure 5: Photostationary state
The photolysis of NO2 produces oxgen atoms that react very quickly with molecular oxygen
O3 reacts very fast with NO to form NO2.
The three reactions form (during sunlight) an equilibrium (that depends on the intensity of sunlight), called photostationary state.
The reactions involved are fast and therefore the photostationary state is reached within minutes.
The photostationary state does not lead to a photochemical net production of O3.
|
Figure 6. UV-spectrum and quantum yields of NO2 |
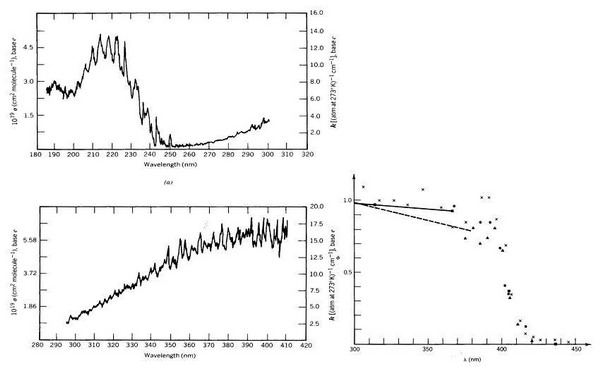 |
Notes to Figure 6 (from Finlayson-Pitts and Pitts, 1986, p. 150 and 154): NO2-photolysis in the troposphere
The absorption spectrum of NO2 (left side) in troposphere is only relevant above approximately 300 nm because the solar light quanta with higher energy are absorbed in the stratosphere (comp. Fig. 1 and 2).
The quantum yield of NO2-photolysis to form NO (right side) is close to one below approximately 390 nm and decreases rapidly when wavelengths are becoming larger.
In the tropopshere the wavelength range from approximately 300 to 400 nm determines the photolysis of NO2 (however, photolysis of NO2 is not rate determing for tropospheric photo chmistry)
2.2. ROx-radical chain
|
Initiation: formation of HOx-radicals by photolysis OH: „cleansing agent“ of troposphere, „oxidation capacity“
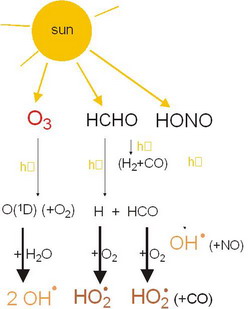
left: Figure 7 |
Notes to Figure 7
Figure 7 shows the dominating photolysis reactions that form reactive HOx radicals, the photolysis of O3 is usually the most important.
The formation of OH from HONO is only important under special conditions.
HO2 is converted to OH (see below).
The OH radical is very reactive, oxidizing most gaseous reactive compounds in the troposphere. OH radicals are therefore called the „cleansing agent“ of the troposphere, and they are the most important contributers to the tropospheric oxidation capacity.
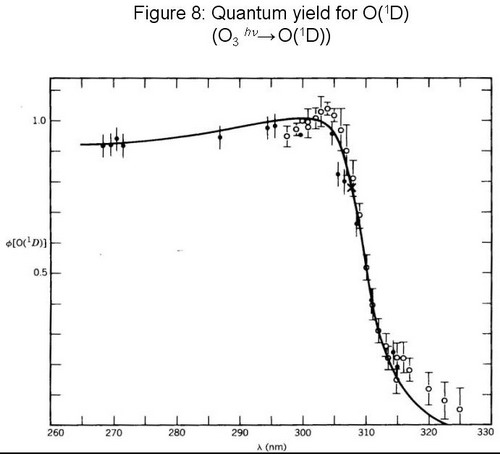 |
Notes to Figure 8 (from Finlayson-Pitts and Pitts, 1986, p. 147):
Ozone has several absorption bands in the UV, visible and IR. OH radicals are only formed from excited oxygen atoms (O(1D)).
The quantum yield to form O(1D) is close to unity around 300 nm but decreases rapidly with wavelengths larger than 305 nm and therefore only a small wavelength band around 300 to 325 nm is important for OH radical formation.
Only a part of O(1D) reacts with H2O, the other part reacts mostly with unreactive molecules such as N2 forming O-atoms in the ground state which react with molecular oxygen to form O3 (see Fig. 5). The humidity in air is therefore an important parameter for the production of OH-radicals.
|
Figure 9. Propagation exemplified by ethane (Volatile Organic Compound (VOC)) |
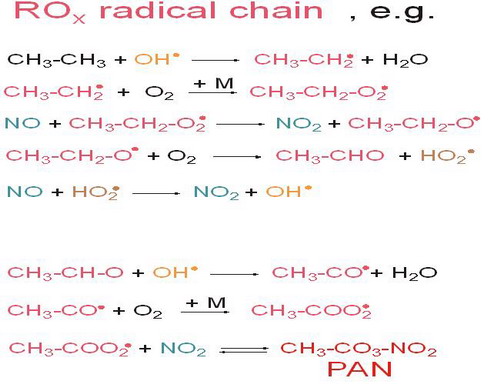 |
Notes to Figure 9: ROx radical chain propagation
A very large number of organic compounds are produced by industry and a large number is emitted from biogenic sources. Their degradation pathways in the troposphere are very complex.
The main reactions of the ROx-radical chain in the troposphere can be characterized by the reactions shown in Fig. 4.9, exemplified by ethane.
Organic species first react with OH radicals forming new radicals that very quickly add O2 to form organic peroxy-radicals.
In the polluted troposphere they react with NO producing organic oxy-radicals and NO2.. NO2 subsequently photolyses leading to O3 formation (see Fig. 4.5).
The oxy-radicals react further with O2 to form aldehydes and HO2, which react in a similar way as organic peroxy-radicals (reacting with NO to form NO2 and OH). By this reaction sequence OH is formed again, yielding a chain reaction.
Because of this chain reaction OH can oxidize most organic compounds efficiently despite the fact that OH concentrations are allways very low in the troposphere.
The formed acetaldehydes react with OH (note that carbonlys can be also photolysed depending on their absorption spectra). If the produced acetylperoxy radical reacts with NO2 Peroxyacetylnitrate (PAN) molecules can be formed. PAN is very phytotoxic and it can be thermally degraded again. PAN reacts as an important reservoir species, which binds a reactive ROx radical with an NO2 radical in polluted air. After transport over large distances PAN can release the reactive radicals again, leading to photooxidant formations thousands of kilometers from the source region of the air pollutants. Note that the thermal stability of PAN is such that the transport in the cold upper troposphere is particularly efficient.
Notes II to Figure 9: Terminology of organic compounds in tropospheric chemistry
VOC: Volatile Organic Compound. VOCs include not only reactive compounds but also compounds such as CFCs that are not relevant for photooxidation.
NM-VOC: Non methane VOC(s), which excludes methane. Methane is much less reactive toward OH than the other organic compounds and therefore not important for photooxidant pollution on regional scales.
ROG: Reactive Organic Compound(s), which most precisely describes the compounds important in photo-oxidation on local and regional scales.
HC: Hydrocarbon(s). Hydrocarbons are the most important precursors for photooxidant formation.
NM-HC: Non methane Hydrocarbon(s), meaning hydrocarbons without methane.
2.3. Most important termination reactions
|
Figure 10. |
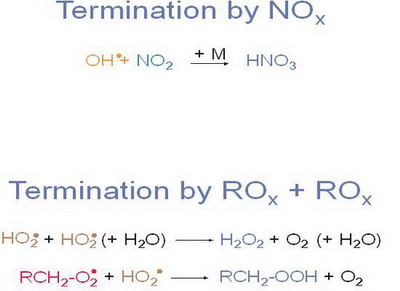 |
Notes to Figure 10
Two types of termination reactions are most important in photooxidation on local and regional scales.
One type of termination reactions only includes radicals of the ROx-chain forming (non radical) hydrogen peroxide or organic peroxides,
the other important termination reaction links the NOx with the ROx radical chain forming HNO3.
The dominance of the termination reaction depends on the concentration of the respective radicals. The formation of HNO3 is more important in strongly polluted air, while the formation of peroxides is more important in less polluted air.
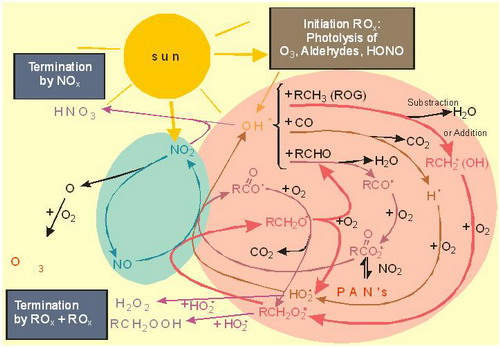
|
Figure 11. Overview of photochemistry in the polluted planetary boundary layer (from Staehelin et al., 2000) |
 |
|
Figure 12. Terms |
 |
2.4. Ozone destruction in (very) clean air (NO less than 10 ppt) exemplified by CO
|
Figure 13. |
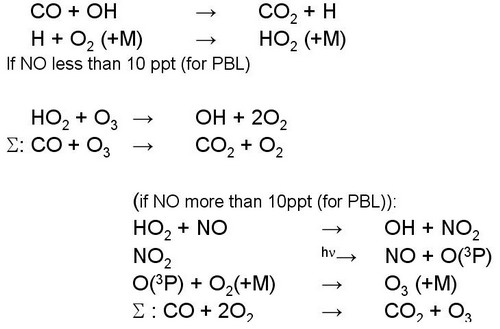 |
Notes to Figure13
Note that Fig.11 does not cover all conditions important in tropospheric chemistry: In case of very low NOx concentrations (around 10 ppt for typical planetary boundary layer condition) ozone is chemically destroyed.
Fig. 13 contains in the first two lines the oxidation of CO to CO2 initiated by OH.
In case of NO concentrations above around 10 ppt NO reacts (dominantly) with HO2 to form NO2 (subsequently leading to O3 as shown in Fig. 11) and OH. If NO concentrations are very low, HO2 reacts with (destroys) O3 to form OH.
Summary and additional remarks
OH is very reactive and OH is the most important oxidation agent for most gaseous pollutants in tropospheric air.
In presence of NOx (NO larger than 10 ppt): Photooxidants (O3, PAN, HNO3, etc.) are formed.
In case of very clean condition (NO smaller than 10 ppt and typical plantery boundary layer O3 concentration): Ozone destruction occurs.
Organic chemistry is only presented in a simplified way in Fig. 11. Tropospheric organic chemistry is very complex (e.g. the reaction of alkenes with O3 is an additional source of HOx).
Ozone precursors (NOx, Volatile Organic Compounds (VOC) and CO) are of anthropogenic or biogenic origin.
3. Oxidation during night
|
Figure 14. |
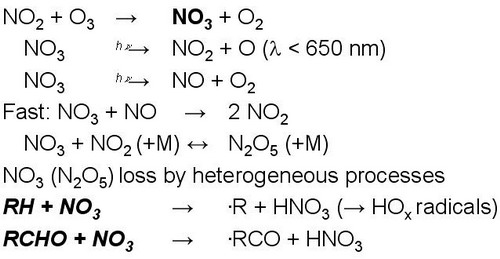 |
In the absence of sunlight, e.g. during night, no photolyses reactions take place which drive the photochemistry shown in Fig. 11.
However, some gas phase oxidation still can proceed via the NO3 radical.
NO3 is produced from reaction of NO2 with O3 (this reaction also proceeds during day, but NO3 is rapidly photolysed because of its strong absoprtion in the visible spectrum and therefore NO3 is not a significant oxidant during the day).
NO3 reacts fast with NO and NO2 which limits NO3 concentrations.
NO3 is a strong oxidant reacting with some organic compounds in a somewhat similar way as OH radicals.
NO3 only reacts fast with specific compounds, which is different to OH.
Oxidants in tropospheric gas phase chemistry
In the presence of solar radiation: OH is the most important oxidant for most gaseous compounds (concentrations strongly depend on pollution level, global mean concentrations approximately 106 cm-3).
In absence of solar radiation (i.e. during night) NO3 is an important oxidant for specific compounds (concentrations are strongly variable, mean concentrations during night approximately 5 108 cm-3 ).
Both, during day and night compounds can be oxidized by O3 (mean value approximately 1012 cm-3 ). However, this reaction is competing with OH and NO3 oxidation only under specific condition for a few compounds (e.g. some reactive alkenes).
A few compounds with strong absorption bands above 300nm and in the visible are photolysed in the troposphere (such as NO2, O3 and carbonyls).
4. Limitation regimes
|
Figure 15. EKMA approach (from Finlayson-Pitts and Pitts, 1986, p. 611) |
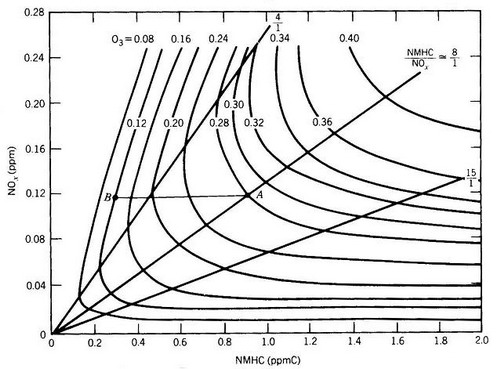 |
Notes I to Fig. 15: The EKMA approach
In order to determine an optimal air pollution abatment strategy to reduce elevated ozone concentrations down wind of strong pollution sources the relation between emission of ozone precursors (nitrogen oxides and organic compounds) has been studied since decades.
One (simplified) approach is to calculate O3-isopleths using EKMA (Empirical Kinetic Modeling approach). The O3 isopleths (e.g. daily ozone maxima, largest values in the upper right corner) are depicted as function of the primary air pollutants in the source region (y-axis: NOx; x-axis: Organic compounds (as ppmC NMHC (C-atoms of non-methane hydrocrabons summed up as volume mixing ratio)).
In addition to the ozone formation from its precursors dry deposition (see Chapter 5.2.) needs to be included as most important sink when caluculating O3 concenctrations in ambient air.
In the classical EKMA approach the calculations are based on chemical box models which calculate tropospheric ozone concentrations along an air parcel as function of travelling time of the air parcel along a trajectory simulating the chemistry shown in Fig. 11. Such box-models ignore any specific mixing effects which might occur during transport. They are repeated many times starting from different initial air pollutant concentrations in the source region which allows to show the results as plots shown in Fig. 15.
The surprising results are shown in the left side of Fig. 15 (consider ispoleths with low O3 isopleths): If NOx concentrations are higher at the emission site, O3 concentrations reaching the receptor site are lower than if the air parcel is loaded by lower NOx concentration.
Notes II to Fig. 15: Interpretation of EKMA diagrams (comp. Fig.11)
In the following we only consider chemsitry (in reality the system is more complex because of planetary boundary layer meteorology which is adressed in chapt. 4.5). In the following argumentation we also assume (i) that the production of OH radicals by initiation reactions (see Fig. 4.7) to be constant and that no additional emission sources (except those of a point source) change the pollutant concentrations in the air parcel.
OH radicals can react (i) with organic compounds leading to peroxyradical formation (see red pathway in Fig. 4.11) which produces O3 by oxidizing NO to NO2 or (ii) OH can react with NO2 forming HNO3 which is a termination reaction supressing O3 formation (blue in Fig. 4.11).
The dominance of pathway (i) over (ii) depends on the NO2 concentration versus the sum of NM-HC concentrations in the air parcel (weigthened over the reaction rates of the individual species).
In urban environments NO2 concentration are usually that large that HNO3 formation dominates the reactions of OH radicals (pathway (ii)), which implies that local O3 production is small. These conditions are also called VOC-limitation because O3 production increases with increasing VOC concentration.
During the next hours when the air parcel might move along the trajectory from an urban to a suburban environment, NO2 concentration in the air parcel steadily decreases because NO2 reacts with the available OH radicals. The decrease in NO2 changes the dominance of pathways (ii) over (i) favouring more pathway (i) and therefore local O3 production increases.
When NOx concentration is decreasing steadily the mixture of organic vs. NOx concentration passes through a state in which the ratio of ozone precursor concentration is such that local O3 production maximizes, which is called the transition regime .
When NOx concentration is decraesing further (by pathway (ii)) local O3 production rate becomes limited by the availibilty of NOx concentration, a regime which is called NOx-limitiation . Such conditions usually occur in rural environments.
|
Figure 16. Development of an urban photo oxidant plume, including VOC(HC)- and NOx- limitation (EMEP, 2004) |
 |
Summary: Typical sequence of chemical regimes of an air parcel loaded by ozone precursors („ageing of air mass“) (Fig. 16)
1. Photostationary state (fast) (see Fig. 5)
2. VOC-limitation: O3 production increases with (increasing) VOC concentration (decreases with increasing NOx) (see Fig.15)
3. Transition regime: Maximum ozone production (see Fig. 15)
4. NOx-limitation: Ozone production increases with NOx concentration (see Fig. 15)
5. Ozone destruction (see Fig. 13)
5. Maximal O3 concentrations reported from PBL in urban plumes
|
Figure 17: North and Central America (from Staehelin, 2002, ext.) |
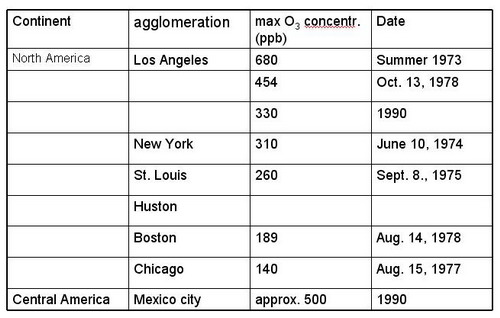 |
|
Figure 17, cont. Maximal O3 concentrations reported from PBL in urban plumes (cont.): Europe, Asia, South America (from Staehelin, 2002, extended) |
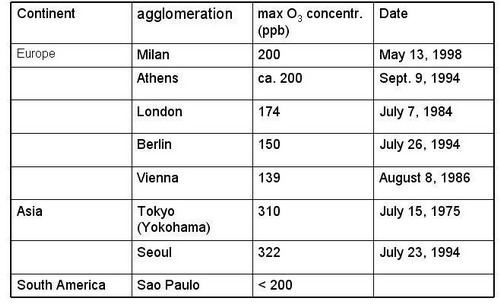 |
Notes to Figure 17
The list of recorded highest O3 concentration is problematic; its representativeness is questionable because (a) O3 monitoring is often not performed in the outflow of agglomerations where highest O3 occur and (b) systematic monitoring usually starts when air pollution is accepted as air pollution problem in the public. Nevertheless, some characteristics seem to be robust
Maximal O3 concentration depends on the emission strength of ozone precursors, ventilation and solar irradiance
In the outflow of large agglomerations elevated O3 concentrations occur all over the world, and some relation between the population size of the agglomeration and maximal O3 concentrations seems obvious
Highest concentrations were reported from the Los Angeles basin in the 1970s and very large concentrations occurred in Tokyo during the 1970s
In industrialized countries ozone maxima in the outflow of agglomerations show a decreasing tendency, due to decrease in emissions of ozone precursors
Today largest O3 concentrations occur in the outflow of the third world „mega cities“. The most famous example is Mexico city, where largest concentrations were measured in the early 1990s. Financial resources to limit emissions of air pollutant might not be available; photo oxidant pollution might increase in future
References
- EMEP, Assessment Part I, European Perspective, EMEP, Oslo, 2004.- Finlayson-Pitts, B. and Pitts, J.N.Jr., Atmospheric Chemistry, John Wiley and Sons, New York, 1986.
- Seinfeld, J. H., and Pandis, S. N., Atmospheric Chemistry and Physics, John Wiley and Sons, New York, 1998.
- Staehelin, J., Prevot, A.S.H., and Barnes, J: Photochemie der Troposphare, Handbuch der Umweltveranderungen und Okotoxikologie, Band IA: Atmosphare, R. Guderian, Ed., Springer Verlag, 207-341 (2000).
- J. Staehelin: Ozone Measurements and Trends (Troposphere), in Encyclopedia of Physical Science and Technology, Thrid Edition, Vol 11, 539-561 (2002).